What is rust? Rust is not just a surface stain on metal — it is a destructive chemical process that weakens strength, shortens product life, and causes massive industrial losses worldwide.
This happens because rust forms when iron reacts with oxygen and moisture, creating iron oxide that slowly eats into metal structures from the inside out. Over time, this oxidation reduces durability, load capacity, and safety.
For example, untreated steel components exposed to humid environments can lose a significant portion of their mechanical strength, leading to cracks, failures, and costly replacements.
That’s why truly understanding what is rust — how it forms, what it’s made of, and how it can be controlled — is essential for anyone working with metal products or manufacturing.
🧱 What Is Rust? A Clear Definition
What is rust? Rust is a reddish-brown flaky substance that forms on the surface of iron or steel when it undergoes a chemical reaction with oxygen and moisture. Scientifically, rust is known as iron oxide, and it appears when iron reacts with water (H₂O) and oxygen (O₂) in the environment.
This process is a classic example of corrosion, but specifically, it refers to the corrosion of iron and its alloys. While all rust is corrosion, not all corrosion is rust — this is an important distinction in material science.
Rust typically starts as small surface spots, but without treatment, it spreads and penetrates deeper, leading to structural weakness. In manufacturing or construction, that means cracks, brittleness, and eventual failure of parts. In essence, rust is both a chemical warning sign and a physical threat to metal products.
Rust doesn’t appear overnight. It often results from long-term exposure to humid air, water, salt, or acidic environments — which are common in many industrial and outdoor applications. If you’ve ever seen a red-brown stain on a metal fence, tool, pipe, or casting part, you’ve seen rust at work.
Rust is not a single substance, but a mixture of iron oxides, typically including Fe₂O₃ (iron(III) oxide) and FeO(OH) (iron(III) oxyhydroxide), depending on the environment.

Importantly, rust is porous and non-protective — unlike the patina that forms on copper or aluminum oxides, which can shield the surface from further corrosion, rust accelerates degradation by trapping moisture and oxygen, making the situation worse over time.
From a manufacturing perspective, rust is the enemy of efficiency. It compromises product integrity, increases maintenance costs, and poses safety risks if not addressed early. That’s why knowing what rust is — not just in appearance but in chemical behavior — is fundamental in industries that rely on metal fabrication.
⚗️ What Is Rust Made Of? The Chemistry and Formula Explained
To understand what is rust, we must look closely at its chemical composition. Rust is the result of a chemical reaction between iron, water, and oxygen. The product of this reaction is a group of compounds collectively known as iron oxides, and they form when iron atoms lose electrons through oxidation.
Rust is not a pure substance. It is a loose, porous mixture of iron(III) oxide (Fe₂O₃) and iron(III) oxyhydroxide (FeO(OH)), often combined with varying amounts of water molecules. The general chemical formula commonly used to describe rust is:
Fe₂O₃·nH₂O
This stands for hydrated iron(III) oxide, where n can vary depending on environmental conditions such as humidity. The rusting process also frequently produces FeO(OH), especially in moist or salty environments. These compounds are all the result of iron being exposed to both oxygen and water over time.
The Chemical Reaction Behind Rust
The formation of rust is a classic redox reaction — meaning it involves both reduction and oxidation steps. Here’s how the process happens:
- Iron oxidizes, losing electrons:
- Fe → Fe²⁺ + 2e⁻
- The free electrons are picked up by oxygen in the presence of water:
- O₂ + 4e⁻ + 2H₂O → 4OH⁻
- The iron ions (Fe²⁺ and Fe³⁺) then react with the hydroxide ions (OH⁻) to form compounds like Fe(OH)₃, which eventually dehydrate to become Fe₂O₃·nH₂O — rust.
So, when someone asks, what is rust made of, the accurate answer is:
➡️ A hydrated mixture of iron oxides, formed by electrochemical oxidation of iron in the presence of moisture and oxygen.
These compounds do not bond tightly to the underlying metal. In fact, they flake off easily, exposing more iron to the air — which means the rusting process is self-perpetuating unless preventive measures are taken.
🎨 What Color Is Rust and Why?
At first glance, most people recognize rust by its distinct reddish-brown appearance. But what is rust, really, in terms of color? And why does it appear that way?
The typical rust color — a mix of red, orange, and brown tones — comes from the iron oxides that form when iron reacts with oxygen and moisture. These compounds reflect light in a way that produces that well-known rusty hue.
The most common compound in rust is iron(III) oxide, also known as Fe₂O₃. This substance has a natural reddish-brown color, which is why most rusted metals appear in that shade. When we ask what is rust, we are not only describing a chemical change, but also a visible transformation — one that signals corrosion and damage.
However, rust is not always the same color. Its appearance can vary depending on environmental conditions and the specific compounds formed during the oxidation process. Here are the most frequently seen rust colors and their causes:
- Reddish-Brown – The classic color, caused by Fe₂O₃·nH₂O (hydrated iron(III) oxide)
- Yellow or Orange – Early-stage rust, or rust formed in high humidity
- Dark Brown or Black – Indicates limited oxygen, often found in underwater or enclosed spaces
- Green or Blue Tint – If copper is present in the alloy, patinas may mix with rust
The texture of rust also affects how we perceive its color. Flaky rust reflects light differently than smooth rust, sometimes appearing darker or more orange depending on how thick the oxide layer is.
Another key point is that what rust looks like can influence decisions in manufacturing and quality control. For instance, surface rust may appear light orange and dusty, signaling an early stage of corrosion. Deeper, darker rust usually indicates structural damage underneath.

So when someone asks what is rust, it’s not just about chemistry — it’s also about color signals. The color of rust tells us how far corrosion has progressed, what environment it’s been exposed to, and what kind of damage might be occurring.
In industrial production, knowing what color rust is helps workers and inspectors assess risk visually and take action before it’s too late. Rust isn’t just ugly — it’s a warning sign that the metal is actively degrading.
🔧 What Is Rust Used For in Practice?
At first, it might sound strange to ask what is rust used for, especially since rust is often seen as a destructive force. But in certain fields and applications, rust — or more accurately, iron oxides — does have practical uses, especially when properly controlled and applied.
In other words, while the process behind what is rust is typically unwanted, the material that results from it can be intentionally used in several industries.
1. 🔬 Pigments and Paints
One of the most common uses of rust — specifically iron(III) oxide (Fe₂O₃) — is as a pigment in paints, coatings, and ceramics.
Known as red iron oxide, this pigment has been used for centuries in:
- Industrial paint coatings
- Art materials and earth-toned pigments
- Road surface coloring
- Brick and concrete tinting
It provides a deep, rich color and excellent UV resistance, making it ideal for outdoor and heavy-duty applications. So ironically, the very thing that destroys metal is also used to protect and beautify other surfaces.
2. 🧲 Magnetic Materials
Certain forms of rust (iron oxides) such as magnetite (Fe₃O₄) have magnetic properties and are used in:
- Data storage (magnetic tapes and disks)
- Magnetic inks in security printing
- Ferrite cores in electronics
Though not exactly the flaky rust seen on metal surfaces, these are part of the same iron oxide family — again demonstrating how understanding what is rust can unlock functional industrial value.
3. 🩻 Medical and Biotech Applications
Iron oxides derived from rust reactions are also used in medicine, especially in imaging and diagnostics. For instance:
- Iron oxide nanoparticles are used in MRI contrast agents
- Targeted drug delivery systems
- Biocompatible coatings for implants
These applications require ultra-pure, synthetic rust compounds, not naturally formed corrosion — but the chemical foundation remains the same.
4. ♻️ Waste Filtration and Environmental Cleanup
Because iron oxides are chemically active, they are used in environmental engineering for:
- Arsenic removal in water treatment
- Heavy metal adsorption from contaminated soil
- Catalysts in industrial chemical reactions
In this context, rust isn’t just a problem — it becomes a solution to global issues like pollution control and resource recovery.
So when we ask, what is rust used for, the answer isn’t “nothing.” While it’s destructive in the wrong place, rust and its chemical cousins have been repurposed by scientists and engineers into valuable industrial materials.
Understanding what is rust, therefore, isn’t just about prevention — it’s also about innovation, and how we can turn corrosion into a controlled, useful material in the right hands.

🧱 What Is Surface Rust vs Deep Rust?
When people ask what is rust, they usually think of any reddish-brown stain on metal. But in manufacturing and industrial inspection, it’s important to distinguish between surface rust and deep rust — because each tells a very different story about the condition of the metal and the urgency of action required.
What Is Surface Rust?
Surface rust is the earliest and most common stage of rust formation. It typically appears as:
- Thin, dusty reddish-orange patches
- Flaky coating that can be easily scraped off
- No visible pitting or damage to the underlying metal
Surface rust is usually caused by brief or light exposure to moisture, such as condensation, humid air, or accidental splashes. It forms quickly — sometimes within hours — especially on raw steel or uncoated iron surfaces.
But here’s the good news: surface rust is mostly cosmetic. It doesn’t yet compromise structural integrity. If identified early, it can often be:
- Brushed or blasted off easily
- Treated with rust converters or primers
- Prevented from returning with proper coatings or sealing
That’s why, when we assess what is rust, understanding its depth and behavior is crucial for deciding next steps.
What Is Deep Rust?
Deep rust is a more advanced and dangerous stage. It occurs when rust is allowed to penetrate beyond the surface layer and into the metal itself.
Here’s how you can recognize deep rust:
- Pitting or holes in the metal surface
- Thick, crusty rust buildup that cannot be brushed off
- Soft or brittle areas in the metal
- Reduced mechanical strength
Deep rust physically eats away at the metal, reducing its thickness and load-bearing capacity. In industries like construction, automotive, or mining equipment, this can lead to:
- Cracks under stress
- Mechanical failure
- Safety hazards
In most cases, once deep rust has set in, the affected part needs replacement or significant rework — cleaning alone is not enough.
Need Help? We’re Here for You!
🛡️ What Metal Doesn’t Rust? Rust-Proof Metals
When we explore what is rust, we’re usually referring to the corrosion of iron and steel. But not all metals rust — in fact, some are completely resistant to rusting because they don’t contain iron at all. Understanding which metals don’t rust is essential in selecting the right materials for corrosion-sensitive environments.
Why Some Metals Don’t Rust
Rust specifically forms through a reaction between iron, water, and oxygen. Therefore, any metal that does not contain iron will not rust. That doesn’t mean it’s immune to all types of corrosion — but it will never produce rust in the traditional sense.
This distinction is key when evaluating materials in industrial design, engineering, or purchasing. Knowing what is rust, and which metals are immune to it, can guide better long-term material choices.
Common Rust-Proof Metals
Here are the top materials that do not rust:
🧲 1. Stainless Steel
Stainless steel contains iron, but it also includes chromium (at least 10.5%) which reacts with oxygen to form a passive oxide layer. This protective layer prevents rust from forming on the surface — as long as it’s not damaged or worn away.
Stainless steel is widely used in:
- Food processing equipment
- Construction and architecture
- Marine components
- Automotive parts
When customers ask what is rust, they’re often surprised that a steel product can be rust-resistant — but it’s all about alloy composition and surface treatment.

🪙 2. Aluminum
Aluminum doesn’t rust because it doesn’t contain iron. Instead, it forms a thin layer of aluminum oxide (Al₂O₃) that protects the metal from further oxidation. This makes aluminum ideal for:
- Aircraft structures
- Window frames
- Outdoor signage
- Consumer electronics
Despite being soft and lightweight, aluminum’s natural corrosion resistance makes it a powerful material where rust must be avoided.
🧱 3. Copper, Brass, and Bronze
These metals do not rust, but they do tarnish or develop a surface patina over time. Unlike rust, this layer is protective, not destructive.
For example:
- Copper turns green (verdigris) in the open air
- Brass and bronze develop a dark brown sheen
These metals are common in:
- Plumbing fixtures
- Decorative architecture
- Electrical systems
Understanding what is rust means knowing how it’s different from other types of oxidation — and why materials like copper corrode differently but much more slowly.
🧪 4. Titanium
Titanium is a high-performance metal that resists corrosion in extremely harsh environments, including seawater, chemicals, and even acid. It doesn’t rust and forms a stable oxide layer that never flakes.
It’s used in:
- Aerospace engineering
- Medical implants
- High-end marine components
When rust resistance is mission-critical, titanium is unmatched.
⚖️ Rust vs Tarnish vs Corrosion: Key Differences
To fully understand what is rust, we must compare it with two commonly confused terms: tarnish and corrosion. These terms are often used interchangeably, but in material science and manufacturing, they refer to distinct chemical processes with very different outcomes.
Let’s break down the differences to clarify what is rust and why it matters so much more in certain applications.
🔧 What Is Corrosion?
Corrosion is the broad, general term for the degradation of metal due to chemical or electrochemical reactions with the environment. It includes:
- Rust on iron or steel
- Patina on copper
- Tarnish on silver
- Oxidation on aluminum
So when someone asks what is rust, the most accurate answer is:
Rust is a specific type of corrosion that occurs only to iron or iron-based alloys like steel.
In short: all rust is corrosion, but not all corrosion is rust.

🧪 What Is Rust (Compared to Other Corrosion)?
Rust is the result of a redox reaction involving iron, oxygen, and water. It produces iron oxides like Fe₂O₃·nH₂O — a flaky, reddish-brown substance that forms on iron or steel surfaces.
The key features of rust include:
- Only forms on ferrous metals (metals containing iron)
- Requires moisture and oxygen
- Weakens the metal by flaking and expanding
- Spreads over time if untreated
That’s why, in industrial contexts, understanding what is rust helps engineers and buyers assess not just appearance, but structural integrity and performance risk.
✨ What Is Tarnish?
Tarnish is a type of surface-level corrosion, but it behaves very differently than rust. Tarnish:
- Occurs on non-ferrous metals, such as silver, copper, or brass
- Forms a thin, dull film — usually gray, brown, or black
- Does not flake or spread, and can often be polished away easily
Tarnish is caused by exposure to sulfur compounds or oxygen in the air, but it doesn’t destroy the metal. In fact, it can sometimes act as a protective layer, slowing further corrosion.
Unlike rust, tarnish doesn’t reduce the strength of the metal — it mostly affects appearance. So when comparing what is rust to tarnish, the difference lies in depth and damage.
🛠️ How to Prevent Rusting of Metals
Now that we’ve fully answered what is rust, it’s time to face the next critical question: how do we stop it from happening?
Understanding what is rust — a destructive chemical process involving iron, moisture, and oxygen — allows us to take targeted actions to prevent it. In metal manufacturing, rust prevention isn’t optional; it’s a fundamental part of ensuring product quality, lifespan, and safety.
Here are the most effective and widely adopted strategies to prevent rusting of metal parts.
🧴 1. Protective Coatings
Applying a physical barrier between the metal surface and the environment is one of the most common ways to stop rust.
- Paints and primers: Create a sealed surface that prevents oxygen and moisture contact.
- Powder coating: Offers a thicker, more durable finish resistant to chipping.
- Zinc-rich primers: Especially useful for steel, these provide sacrificial protection (galvanic).
In our factory, we often recommend clients specify coating types in advance — especially for products used outdoors, near water, or in humid climates. Once you understand what is rust, coating becomes a first line of defense.
🔩 2. Galvanization
Galvanization is the process of coating iron or steel with zinc. The zinc corrodes first, protecting the base metal beneath. This is known as sacrificial protection.
- Hot-dip galvanizing is ideal for heavy-duty applications like construction.
- Electro-galvanizing offers a cleaner, thinner finish for precise parts.
Because zinc reacts more readily than iron, it helps prevent the very process that causes rust. If you know what is rust, you’ll appreciate why zinc is so valuable in metal finishing.
🧪 3. Use of Rust Inhibitors
Rust inhibitors are chemical treatments applied to metal surfaces to slow or block oxidation.
- Often used in machining and storage environments
- Sprayed, brushed, or added to lubricants
- Some are temporary (for shipment), while others are permanent (like phosphating)
These solutions are particularly useful when shipping overseas, where temperature changes can cause condensation inside containers — a common trigger for rust.
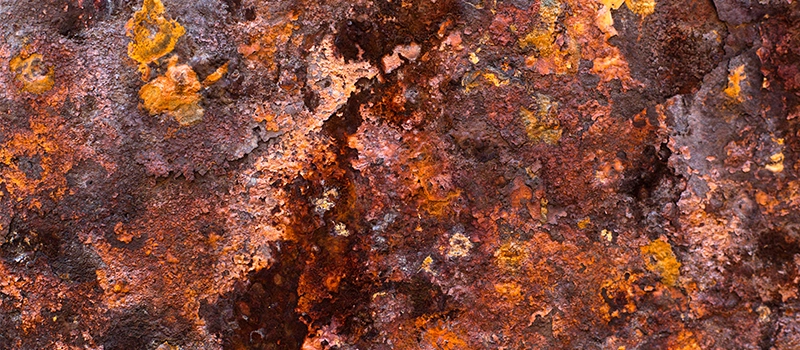
🌬️ 4. Environmental Control
Another way to prevent rust is by controlling the environment where metals are stored or used:
- Keep humidity below 50%
- Use desiccants in packaging
- Apply VCI (Vapor Corrosion Inhibitor) paper during shipment
Even small details — like avoiding direct contact with concrete floors — can help. If you understand what is rust, you understand that moisture is the enemy.
🔄 5. Regular Maintenance and Inspection
No matter how rust-resistant a material is, nothing beats routine checks:
- Clean off dirt, salt, or moisture build-up
- Touch up damaged coatings
- Reapply inhibitors in high-risk environments
Factories that ignore rust prevention often face massive losses later. That’s why knowing what is rust must be paired with a prevention plan.
✅ Conclusion
What is rust is not just a simple question — it’s the gateway to understanding how metals fail, how industries lose billions, and how manufacturers like us can take smart, preventive action.
By learning what rust is made of, how it forms, what it looks like, and how to stop it, we give ourselves the tools to make better choices — in materials, processes, and long-term performance. Whether you’re in metal sourcing, product design, or industrial maintenance, understanding what is rust isn’t optional — it’s essential.








