Have you ever faced quality issues in your casting projects and wondered if temperature control was the real culprit? Are your parts deforming, failing under heat, or showing cracks during machining? The root cause might be a lack of understanding about the iron melting point.
Getting the iron melting point wrong can result in serious problems—delays in production, increased defect rates, and rising costs. Whether you’re working with pure iron, cast iron, or an iron alloy, the precise iron melting point plays a vital role in shaping performance and durability. Overlooking it risks structural weakness, poor tolerances, and even total part failure.
In this article, we’ll explore the iron melting point in detail—what it is, why it matters, and how it affects real-world manufacturing processes like casting, forging, and precision machining. You’ll discover how temperature influences iron’s transformation and learn how mastering the iron melting point can improve your product quality, efficiency, and bottom line.
What Is the Iron Melting Point?
Definition of Melting Point in Metallurgy
In metallurgy, the melting point is defined as the temperature at which a metal changes from a solid to a liquid under standard atmospheric pressure. For industrial materials like iron, this transition marks the exact point where heat energy overcomes the atomic bonds holding the crystal structure together.
Unlike organic materials, metals like iron have defined, measurable melting points—a key property for casting, forging, and thermal treatment processes.
The iron melting point in Celsius, Fahrenheit, and Kelvin
The iron melting point is:
- 1,538°C (Celsius)
- 2,800°F (Fahrenheit)
- 1,811 K (Kelvin)
This high temperature makes iron suitable for structural and mechanical applications where heat resistance is critical. Its melting point is significantly higher than many other common metals, which is why iron is frequently chosen for components that operate in extreme thermal conditions.
Scientific Explanation: How Atomic Structure and Bonding Define Melting Behavior
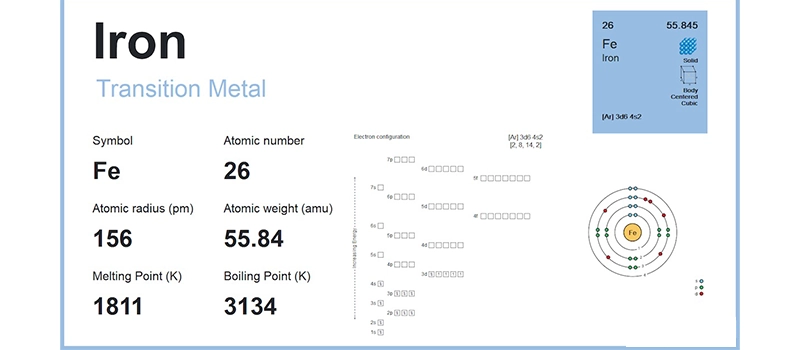
Iron is a transition metal with a body-centered cubic (BCC) crystal structure at room temperature. As temperature increases, its atomic vibrations intensify until the cohesive forces between atoms can no longer maintain the solid lattice.
The strength of the metallic bonds—formed by shared electrons in a delocalized “electron sea”—is what gives iron its high melting point. Breaking these bonds requires substantial energy, which is why iron doesn’t liquefy easily under heat.
The melting behavior of iron is directly related to its atomic number (26), electron configuration ([Ar] 3d⁶ 4s²), and strong metallic bonding, all of which contribute to the energy required to move it from a solid to a liquid phase.
Transition from Solid to Liquid: What Happens at ~1,538°C (2,800°F)
At the melting point, iron transitions sharply from a solid to a liquid. This phase change is physical, not chemical—the atomic identity of iron remains the same, but its molecular arrangement becomes fluid.
This behavior is critical in casting processes. When heated to just above 1,538°C, iron becomes a molten metal that can be poured into molds. Its sharp transition allows for predictable flow and controlled solidification, which is essential for producing precision parts.
During this transition:
- The iron expands slightly
- Viscosity drops
- Heat transfer increases
- Surface tension changes—affecting how it fills mold cavities
Understanding and managing this process helps manufacturers produce components that are dense, defect-free, and dimensionally accurate.
Chemical and Physical Properties of Iron That Influence Its Melting
The iron melting point is not an isolated number. It is the result of deep, interrelated atomic forces and structural characteristics that define how iron behaves under heat. By examining iron’s chemical and physical nature, we can better understand why it melts at such a high and specific temperature.
Iron’s Atomic Configuration and Bonding Forces
Iron’s atomic structure plays a direct role in its melting behavior. With an atomic number of 26, iron has the electron configuration [Ar] 3d⁶ 4s². These outer electrons are loosely held and form what’s known as a metallic bond—a sea of delocalized electrons shared among positively charged metal ions.
This type of bonding is very strong. It holds atoms in a fixed crystal lattice and requires a substantial amount of thermal energy to break. That’s why the iron melting point is so high compared to metals like lead or aluminum, which have weaker bonding and fewer valence electrons.
The strength of these metallic bonds is a key reason why iron remains solid until it reaches 1,538°C. In comparison, aluminum melts at just 660°C—less than half that of iron.
The Role of Electron Structure and Crystal Lattice
At room temperature, iron crystallizes in a body-centered cubic (BCC) lattice. As the temperature rises, it transitions to a face-centered cubic (FCC) structure, which is more densely packed and stable at higher temperatures. This phase change occurs well before melting, allowing iron to adapt to stress and load without immediately deforming.
Eventually, at its melting point, even the FCC structure can no longer hold together. The atoms break free from their fixed positions, and the material becomes molten.
This shift from a solid to a liquid occurs due to the breaking of cohesive forces in the metallic lattice. Iron’s ability to change lattice structures before melting makes it uniquely capable of withstanding mechanical and thermal stress in heavy-duty applications.
Relationship Between Density, Thermal Conductivity, and Melting Behavior
Iron’s density (7.87 g/cm³) and thermal conductivity (approximately 80 W/m·K at room temperature) further influence its behavior near the melting point.
- A high density means more mass per volume, requiring more energy input to raise the temperature.
- High thermal conductivity ensures that heat is distributed quickly and evenly throughout the material.
Together, these factors contribute to uniform heating during melting, preventing localized hot spots that could lead to uneven flow or structural defects.
In engineering, this consistency is essential. Parts made from iron can heat and melt predictably, which is why it remains a favorite for controlled manufacturing environments where temperature precision is non-negotiable.
Factors That Affect the Iron Melting Point
Although the iron melting point is commonly cited as 1,538°C (2,800°F), the actual melting behavior in industrial settings depends on a variety of internal and external factors. Understanding what shifts or disturbs this baseline is essential for controlling casting quality and metallurgical precision.
Impact of Impurities and Ore Quality
The purity of iron has a direct impact on its melting point. Iron obtained from natural ores often contains impurities such as sulfur, phosphorus, and silicon. These elements disrupt the atomic lattice and can shift the iron melting point downward or create inconsistencies during heating.
For example:
- Sulfur promotes brittleness and weakens grain boundaries
- Phosphorus lowers the melting range under certain conditions
- Silicon, when present, changes fluidity and melting dynamics
Maintaining high-purity raw material is critical in stabilizing the iron melting point during industrial processing.
Influence of Carbon and Other Alloying Elements
The addition of carbon and alloying elements modifies the thermal behavior of iron, though their specific effects on melting temperature are addressed in a later section. What matters here is the principle:
When carbon is introduced into the metallic matrix, it alters atomic spacing and bonding strength. This change lowers or adjusts the iron melting point, making the material more responsive to thermal inputs.
Elements such as nickel, chromium, and manganese are commonly used to enhance strength and corrosion resistance—but they also influence how iron melts. Even small adjustments to composition must be factored in when setting process temperatures.

Pressure and Atmospheric Variation During Melting
The standard iron melting point is measured at 1 atm pressure. In real-world processing, however, furnaces may operate in vacuum or pressurized chambers. These environmental variables slightly shift how iron behaves near its melting temperature.
Increased pressure tends to raise the iron melting point by restricting atomic movement. Vacuum conditions can lower the effective melting point, which helps reduce oxidation and energy consumption in high-purity melting systems.
Though the change is subtle, pressure-sensitive applications require precise adjustments to ensure consistency and safety.
Environmental Exposure and Oxidation During Heating
Iron readily oxidizes at high temperatures, forming surface layers of iron oxide. This oxidation:
- Reduces thermal efficiency
- Promotes slag formation
- Alters the flow characteristics of molten iron
When oxidation occurs, it can obscure the true iron melting point or require more energy input to achieve liquefaction. This not only increases cost, but also affects casting surface quality and dimensional stability.
Controlling exposure to oxygen—through inert gas shielding or flux additives—is key to preserving the melting characteristics of iron.
Get a quote now!
Iron Alloys and Their Melting Points
Pure iron has a consistent melting point of 1,538°C (2,800°F), but in industrial applications, iron is rarely used in pure form. Instead, it is alloyed with other elements to improve properties such as strength, corrosion resistance, and castability. These iron alloys each have different compositions—and therefore, different melting behaviors.
Understanding the iron melting point in relation to alloying is essential for selecting the right material for each application and ensuring optimal casting and machining outcomes.
Cast Iron: Gray, White, and Ductile
Cast iron is an iron-carbon alloy with a carbon content typically between 2% and 4%. This elevated carbon level dramatically lowers the melting point compared to pure iron. Instead of a single value, cast iron melts over a range of approximately 1,150°C to 1,200°C.
- Gray cast iron is the most common type, known for excellent fluidity and compressive strength. Its graphite flakes lower the iron melting point and enhance machinability.
- White cast iron contains cementite (iron carbide), making it extremely hard but more brittle. Its melting behavior is similar to gray iron, though cooling rates affect its structure.
- Ductile iron (also called nodular iron) includes magnesium to form graphite nodules, enhancing ductility while maintaining a lowered melting point.
Because of these properties, cast iron is ideal for complex shapes, pressure-bearing components, and vibration-damping parts.
Wrought Iron
Wrought iron is a low-carbon alloy, typically containing less than 0.08% carbon. It is soft, ductile, and resistant to corrosion, often used in historical structures and decorative elements.
The iron melting point of wrought iron is slightly lower than that of pure iron due to trace impurities like slag, phosphorus, and silica. However, the difference is minimal—typically melting around 1,480°C to 1,520°C.
Its fibrous microstructure makes it unsuitable for modern high-strength demands, but it remains historically important and corrosion-resistant in open-air environments.
Steel and Iron-Carbon Alloys
Steel is perhaps the most widely used form of iron in modern industry. With carbon content ranging from 0.02% to 2.0%, its melting range varies depending on composition:
- Low-carbon steel (mild steel): 1,425–1,530°C
- Medium-carbon steel: 1,430–1,500°C
- High-carbon steel: 1,390–1,480°C
As carbon increases, the iron melting point decreases. Alloy steels—those with added elements like chromium, nickel, vanadium, or molybdenum—further alter thermal behavior.
Steel does not melt at a fixed point like pure iron; instead, it melts gradually across a temperature range, requiring tighter process controls during casting or welding.
Galvanized Iron, Iron-Nickel, and Iron-Chromium Systems
These are specialized iron alloys used in advanced or corrosive environments:
- Galvanized iron is coated with zinc. While zinc melts at just 419°C, the underlying iron melting point remains high. However, overheating can damage the coating, releasing fumes and weakening the material.
- Iron-nickel alloys are used in aerospace and electronics for their thermal stability. Nickel raises corrosion resistance and maintains a relatively high melting point (around 1,450–1,500°C).
- Iron-chromium alloys, such as those used in stainless steel, are designed for oxidation resistance and heat endurance. The melting point may range from 1,400–1,500°C depending on the specific grade.
These advanced alloys are essential in applications where standard cast iron or steel cannot meet performance or durability requirements.
Melting Range Chart for Common Iron Alloys
| Alloy Type | Approximate Melting Range (°C) |
|---|---|
| Pure Iron | 1,538 |
| Gray Cast Iron | 1,150–1,200 |
| White Cast Iron | 1,150–1,200 |
| Ductile Iron | 1,150–1,250 |
| Wrought Iron | 1,480–1,520 |
| Low-Carbon Steel | 1,425–1,530 |
| High-Carbon Steel | 1,390–1,480 |
| Stainless Steel | 1,400–1,500 |
| Iron-Nickel Alloy | 1,450–1,500 |
| Galvanized Iron (core) | ~1,500 (zinc melts at 419) |
This table provides an easy reference point when selecting materials for casting, machining, welding, or high-heat applications.
Why Iron’s Melting Point Matters in Modern Casting
In modern manufacturing, casting is a foundational process used to produce metal components of all shapes and sizes. Whether it’s for agricultural machinery, construction parts, or automotive systems, achieving consistent quality begins with one key variable: the iron melting point.
If iron is not heated to the correct temperature, the casting process may result in defects, poor structural integrity, or increased material waste. A precise understanding of the iron melting point helps engineers and technicians avoid these issues while improving process efficiency and final product performance.

Pouring Temperature and Control
To create a clean, complete casting, molten iron must be poured into molds at just the right temperature—usually above the iron melting point, but not excessively so.
- Pouring too cold causes incomplete mold filling, early solidification, or surface roughness.
- Pouring too hot increases oxidation, promotes gas absorption, and can damage mold walls.
For example, gray cast iron is typically poured at 1,350–1,450°C, while steel may require pouring at 1,600°C or higher depending on alloy content. These values are determined by the specific iron melting point and the thermal behavior of the material.
Accurate pouring temperature control ensures:
- Full cavity fill
- Uniform grain structure
- Minimized defects like porosity or cold shuts
Mold Design and Material Selection
The iron melting point directly impacts decisions about mold material and design. Different casting methods—such as sand casting, investment casting, or die casting—require molds that can withstand or manage the high temperatures involved.
- For pure iron or steel, molds must resist temperatures above 1,550°C.
- For cast iron, slightly lower requirements still demand thermal stability and controlled cooling.
A mismatch between the iron’s melting behavior and the mold’s thermal limits can result in erosion, gas reactions, or premature mold failure. Engineers must carefully design molds based on expected molten iron temperatures, solidification rates, and heat transfer requirements.
Cooling Behavior and Shrinkage Management
Once the iron has been poured, cooling and solidification must be carefully managed. As molten iron cools, it shrinks in volume. If this shrinkage is not properly predicted and controlled, the casting may crack, warp, or develop internal voids.
The rate of cooling is directly influenced by:
- Pouring temperature (how far above the iron melting point it is)
- Mold thermal conductivity
- Wall thickness and geometry of the casting
By understanding the melting and solidification behavior, manufacturers can implement strategies like:
- Riser placement for feeding molten metal during shrinkage
- Chills for localized cooling control
- Precise timing of mold removal or shake-out
Shrinkage control is particularly important in components where tolerances and mechanical performance are critical.
Direct Link to Surface Quality and Internal Defects

Many surface and internal casting defects originate from poor thermal control related to the iron melting point. Examples include:
- Cold shuts: Caused by insufficient temperature during flow
- Gas porosity: Often results from overheating and gas entrapment
- Hot tears: Related to uneven cooling and thermal stress
A consistent, well-regulated approach to melting and pouring iron ensures that the casting surface is smooth, structurally sound, and free from unexpected flaws.
Ultimately, a detailed understanding of the iron melting point leads to better casting outcomes, reduced rework, lower scrap rates, and more reliable components across industries.
Real-World Industrial Applications Based on Melting Point
The iron melting point is more than just a thermal property—it’s a key driver behind how, where, and why iron-based materials are selected across global industries. Its high melting temperature enables iron to withstand extreme heat, pressure, and mechanical loads, making it indispensable in demanding applications.
From agriculture to energy, the iron melting point influences design choices, production techniques, and long-term reliability.

Precision Casting for Agriculture, Automotive, and Petrochemical Industries
In sectors like agriculture and automotive, cast iron components are used for their excellent wear resistance and ability to maintain shape under heat. This includes:
- Engine blocks
- Brake drums
- Transmission housings
- Hydraulic pump bodies
- Tractor chassis components
The melting point of iron—and especially of cast iron alloys—allows these parts to be poured into detailed molds at controlled temperatures. High precision and material integrity are essential here, as these parts often experience fluctuating thermal and mechanical loads.
In the petrochemical industry, the high iron melting point ensures that components like valve housings, pipe fittings, and pressure vessels can endure extreme process heat without deformation or failure. Many of these parts operate in environments where steel or aluminum would soften or corrode under sustained thermal stress.
Heat-Resistance Considerations in Design
Designers and material engineers rely on the iron melting point when creating products that must perform in high-temperature environments. This includes:
- Industrial furnaces
- Heat exchangers
- Combustion chambers
- Heavy-duty brake systems
Need Help? We’re Here for You!
Unlike low-melting-point materials like aluminum (660°C) or zinc (419°C), iron and its alloys offer thermal endurance well above 1,000°C, making them far more reliable under continuous operation.
Selecting iron for these components isn’t just about cost—it’s about thermal stability, dimensional reliability, and reduced maintenance. These advantages directly stem from iron’s resistance to softening and distortion at elevated temperatures.
Structural Reliability in Construction and Heavy-Duty Machinery
In construction and infrastructure, cast and forged iron components are used in:
- Bridge supports
- Steel-reinforced concrete structures
- Heavy machinery frames
- Cranes and lifting equipment
These structures demand both load-bearing capacity and resistance to heat, particularly in regions with high ambient temperatures or fire safety requirements.
The iron melting point ensures that, even in cases of short-term high-temperature exposure—such as welding, surface hardening, or fire—iron-based materials retain their core structure without sudden collapse.
Additionally, its predictable thermal expansion and solidification behavior allow engineers to design with tighter tolerances and more confidence in long-term performance.
Whether used in the casting of small mechanical parts or the fabrication of large infrastructure elements, the iron melting point plays a silent but powerful role behind the scenes. It governs material choice, dictates process conditions, and directly influences the reliability of critical components across nearly every industrial sector.
Iron vs. Other Metals: Melting Point Comparison in Engineering Use
When selecting materials for industrial use, one of the most critical factors engineers consider is the melting point. A metal’s ability to withstand high temperatures without losing structural integrity directly affects how and where it can be used. The iron melting point sits in a range that offers a perfect balance between processability and performance—making it a preferred choice in many demanding applications.
To better understand iron’s role, let’s compare it with other commonly used metals across industries.
Side-by-Side Comparison: Melting Points of Common Metals
| Metal | Melting Point (°C) | Melting Point (°F) |
|---|---|---|
| Zinc | 419 | 786 |
| Lead | 327 | 621 |
| Aluminum | 660 | 1,220 |
| Magnesium | 650 | 1,202 |
| Copper | 1,085 | 1,985 |
| Silver | 962 | 1,763 |
| Gold | 1,064 | 1,947 |
| Titanium | 1,668 | 3,034 |
| Iron | 1,538 | 2,800 |
| Steel (alloys) | ~1,370–1,540 | ~2,498–2,804 |
| Nickel | 1,455 | 2,651 |
The iron melting point of 1,538°C is notably higher than many non-ferrous metals, placing it in a category suitable for high-heat applications while still being manageable for standard casting and forging operations.
How Melting Point Affects Use, Performance, and Cost
- Aluminum and magnesium are lightweight and easy to cast but unsuitable for high-temperature environments due to their low melting points.
- Copper and silver have good electrical and thermal conductivity, but their lower melting points limit them in structural applications.
- Gold and silver are stable and corrosion-resistant but are too soft and expensive for structural use.
- Titanium offers superior strength and heat resistance but is costly and difficult to process.
Iron stands out because it combines:
- High thermal stability
- Excellent mechanical properties
- Reasonable cost
- Ease of alloying and fabrication
This makes it ideal for applications that involve heat, friction, load, or chemical exposure—without driving up manufacturing costs.
Why Iron Is Still Preferred in Many High-Temperature Applications

The iron melting point places it in a category of metals that can:
- Withstand sustained high temperatures
- Maintain mechanical strength near its melting limit
- Be cast, forged, and welded with existing industrial infrastructure
For example:
- In automotive brake systems, aluminum components would fail under heat, but cast iron remains stable.
- In industrial furnaces, where temperatures exceed 1,000°C, iron-based linings and parts last longer than copper or zinc alternatives.
- In construction, structural steel (an iron alloy) offers consistent performance even under fire exposure.
Iron’s performance is not only determined by its melting point but enabled by it. It melts high enough to offer durability and load resistance, yet low enough to be cost-effectively melted, cast, and shaped using standard equipment.
This balance makes iron and its alloys the backbone of modern industrial engineering, where performance, economy, and safety must align.
Boiling Point vs. Melting Point of Iron: Why Both Matter
While the iron melting point is commonly discussed in casting and manufacturing, its boiling point is equally important—especially in extreme industrial processes like vacuum refining, high-temperature welding, and aerospace applications. Understanding the difference between melting and boiling points provides deeper insight into iron’s thermal behavior and industrial versatility.
What Is the Boiling Point of Iron? (~2,862°C / 5,204°F)
The boiling point of iron is approximately 2,862°C (or 5,204°F), which is the temperature at which iron transitions from the liquid phase to the gas phase. This temperature is nearly twice as high as the iron melting point, which is 1,538°C.
At the boiling point, iron atoms gain enough energy to escape the surface of the liquid as vapor, overcoming not just intermolecular bonds but also surface tension and atmospheric pressure. This behavior is rarely encountered in standard manufacturing but becomes relevant in specialized processes.

Get a quote now!
Explanation of Phase Transitions: Solid → Liquid → Gas
Iron undergoes two major phase changes with heat:
- Melting (Solid to Liquid):
Occurs at 1,538°C. Atomic bonds loosen, and the rigid structure breaks down into a flowing liquid. - Boiling (Liquid to Gas):
Occurs at 2,862°C. The liquid becomes vapor as atoms break free completely from the surface.
These transitions are physical changes, not chemical ones—iron remains elemental throughout both phases. However, each phase has distinct thermal, mechanical, and flow characteristics that must be controlled in different stages of manufacturing.
Applications in High-Vacuum and Aerospace Metallurgy
In most industrial settings, iron is processed below its boiling point. However, in aerospace, advanced welding, and vacuum metallurgy, operations may approach or utilize conditions near or above the iron melting point, and in rare cases, approach its boiling point.
Key applications include:
- Vacuum induction melting: Used to purify high-performance iron-based alloys. Lower ambient pressure reduces boiling risks and improves material quality.
- Plasma welding and cutting: High-intensity heat sources may locally exceed the iron boiling point, especially in narrow, focused zones.
- Rocket engine and turbine materials: Components must resist degradation even near boiling conditions, making the boiling point a design constraint.
Understanding both the melting point and boiling point of iron helps engineers select materials, control process temperatures, and predict failure mechanisms in thermal extremes.
Clarifying Common Questions About Melting vs. Boiling
Is the melting point the same as the boiling point?
No. The iron melting point marks the transition to a liquid, while the boiling point marks the transition to a gas. The gap between them (over 1,300°C) provides a wide operating window for casting, forming, and heat treating.
Can boiling occur in industrial casting?
Not under normal pressure. Boiling iron would require extreme heat and controlled environments, which are generally avoided due to energy cost and safety risks.
Why is the difference between these points important?
The wide separation allows iron to exist as a liquid across a broad range, enabling precise control in casting, alloying, and thermal treatment without risking vaporization.
By fully understanding both the iron melting point and its boiling point, manufacturers and engineers can confidently operate within safe, efficient temperature zones—delivering products that meet performance standards across the most demanding applications.
Environmental Impact of Ironmaking and Production
Reaching the iron melting point—whether for casting, alloying, or refining—is an energy-intensive process that carries a significant environmental burden if not managed properly. From carbon emissions to slag formation, every stage of iron production contributes to the global ecological footprint. However, as the industry advances, new methods are being implemented to reduce the environmental cost of producing and melting iron.
Understanding these impacts is essential for global buyers and procurement officers seeking not only competitive pricing, but also partners aligned with environmental responsibility and sustainable growth.
High Energy Consumption at the Iron Melting Point
Melting iron at 1,538°C (2,800°F) requires substantial energy input. Traditional fuel-based furnaces (coal, coke, oil) generate high thermal output but also:
- Burn fossil fuels at large scale
- Release greenhouse gases (mainly CO₂ and NOₓ)
- Contribute to thermal inefficiency and heat loss
According to industry estimates, producing 1 metric ton of molten pig iron using blast furnaces can emit over 1.8 tons of CO₂, much of it linked to the heat required to reach and maintain the iron melting point.
Modern electric arc furnaces reduce direct emissions, but overall grid energy consumption still matters—especially in countries where electricity is fossil-fuel derived.
Carbon Emissions and Particulate Pollution
Iron production generates more than just carbon dioxide. As iron is heated and melted, it also releases:
- Particulate matter (PM) from combustion
- Volatile metal oxides, especially during oxidation
- Sulfur dioxide (SO₂) and carbon monoxide (CO) depending on fuel type and ore impurities
These emissions not only affect local air quality but also contribute to global climate change. Facilities without proper emissions control (e.g., scrubbers, baghouse filters) risk significant environmental penalties and regulatory non-compliance.
More manufacturers are now investing in:
- Flue gas cleaning systems
- CO₂ capture and storage (CCS) technology
- Renewable energy for electric furnaces
Such strategies directly reduce the environmental cost of reaching the iron melting point.
Slag Generation and Industrial Waste
When iron is melted—especially in blast or basic oxygen furnaces—non-metallic elements separate to form slag, which must be removed before casting or refining. While some slag can be recycled into cement or road materials, much of it ends up as waste.
Environmental concerns linked to slag include:
- Heavy metal leaching
- Landfill accumulation
- Groundwater contamination risks
Advanced filtration and slag treatment systems now allow for more responsible handling and reuse. In integrated operations, up to 80% of slag can be repurposed—reducing both waste and production costs.
Sustainable Melting Practices and Industry Reforms
The global ironmaking industry is under pressure to reduce emissions without sacrificing thermal control or metal quality. This has given rise to several eco-conscious trends aimed at reducing the footprint of processes associated with the iron melting point:
- Hydrogen-based reduction instead of carbon-rich coke
- Biomass or biochar alternatives for furnace fuel
- Electrification of melting using green energy
- Circular economy practices through scrap recycling
Iron melting powered by renewable electricity—paired with smart automation and AI-based temperature control—can reduce emissions by over 40% compared to traditional fossil-fuel methods.
For environmentally conscious buyers, manufacturers that embrace these innovations offer both performance and sustainability.
Controlling the iron melting point efficiently doesn’t just improve part quality—it reduces energy waste, cuts pollution, and helps align metal production with long-term environmental goals. The future of ironmaking lies in methods that balance precision, productivity, and planetary responsibility.
Innovations in Iron Melting Technology and Processing
Controlling the iron melting point is no longer a basic furnace operation—today, it involves advanced technologies designed to maximize energy efficiency, material precision, and environmental sustainability. As demand increases for cleaner, more consistent production, industries around the world are adopting new melting methods that enhance process reliability and reduce waste.
These innovations not only ensure better control of the iron melting point, but also unlock new applications for iron in high-performance sectors.

Induction Melting, Arc Furnaces, and Energy-Saving Furnaces
Induction melting has become one of the most efficient and precise ways to reach and maintain the iron melting point. It uses electromagnetic currents to heat the metal internally, allowing:
- Faster melting rates
- Precise temperature control
- Cleaner molten iron (minimal contamination)
Electric arc furnaces (EAFs) are commonly used in steelmaking. They utilize high-voltage arcs between graphite electrodes to melt scrap iron and steel at high temperatures. EAFs allow producers to:
- Melt large volumes of material efficiently
- Reach temperatures above the iron melting point quickly
- Control melting atmospheres to reduce oxidation
Energy-efficient furnaces with regenerative burners and smart insulation are also widely used in foundries to reduce heat loss and improve thermal consistency. These systems lower operating costs while maintaining stable melting behavior—an important advantage in price-sensitive markets.
Advanced Temperature Control Systems
Modern melting operations rely on real-time thermal monitoring to ensure that iron reaches and maintains the precise temperature needed to melt efficiently—without overheating. Key innovations include:
- Infrared thermal sensors: Provide accurate, non-contact temperature measurements.
- Thermocouple arrays: Measure temperatures at multiple furnace zones for uniform heating.
- Digital controllers and AI algorithms: Automatically adjust fuel flow, oxygen levels, and heating rates based on real-time data.
These systems not only improve control over the iron melting point, but also help reduce material waste, fuel consumption, and casting defects caused by temperature fluctuations.
Need Help? We’re Here for You!
Smart Automation and Thermal Monitoring
With the integration of Industry 4.0 technologies, many foundries and steel plants are automating their melting lines. These systems track:
- Temperature curves during melting and holding
- Pouring times and rates
- Thermal profiles of molds and cooling stages
Automated thermal control ensures that the iron consistently reaches the required melting point, and remains in the optimal liquid state until casting is complete. This improves:
- Dimensional accuracy
- Metallurgical structure
- Product consistency across batches
Additionally, smart systems can detect anomalies (such as temperature spikes or heat loss) and alert operators before defects occur—reducing downtime and rework costs.
Trends in Green Metallurgy and Low-Emission Processes
As environmental regulations tighten, melting technologies are evolving to reduce the carbon footprint associated with reaching the iron melting point. Key trends include:
- Electric furnaces powered by renewable energy
- Carbon capture systems for exhaust treatment
- Recycling-based melting to reduce raw ore dependency
Some modern iron producers are experimenting with hydrogen-based reduction and plasma arc melting, which can achieve cleaner processing while minimizing CO₂ output.
These innovations represent the future of sustainable iron processing—where the iron melting point is reached with maximum efficiency and minimal environmental impact.
From precision induction melting to AI-powered furnaces, the evolution of iron melting technologies is transforming the way the industry approaches quality, energy use, and environmental responsibility. Controlling the iron melting point with accuracy and consistency is no longer a challenge—it’s a competitive advantage.
Conclusion: Iron’s Melting Point Is Still Shaping Industry
From casting precision to thermal performance, the iron melting point remains a defining factor in the success of modern metal components. It influences every step of production—material selection, mold design, quality control, and long-term durability.
At Dalian ZhongSheng Metal Products, we understand that mastering melting temperature isn’t optional—it’s essential. With advanced equipment, strict temperature controls, and decades of expertise in casting and machining, we deliver iron parts that meet the highest standards of quality, efficiency, and performance.
If you’re looking for a reliable metal manufacturing partner that takes materials science seriously—starting with the melting point—we’re ready to support your next project.








